Solved The compressibility factor, Z, can be thought of as a

Answer to Solved The compressibility factor, Z, can be thought of as a

Compressibility factor (Z) for a van der Waals real gas at critical point is
If Z is a compressibility factor, van der Waals' equation at low pressure can be written as - Sarthaks eConnect
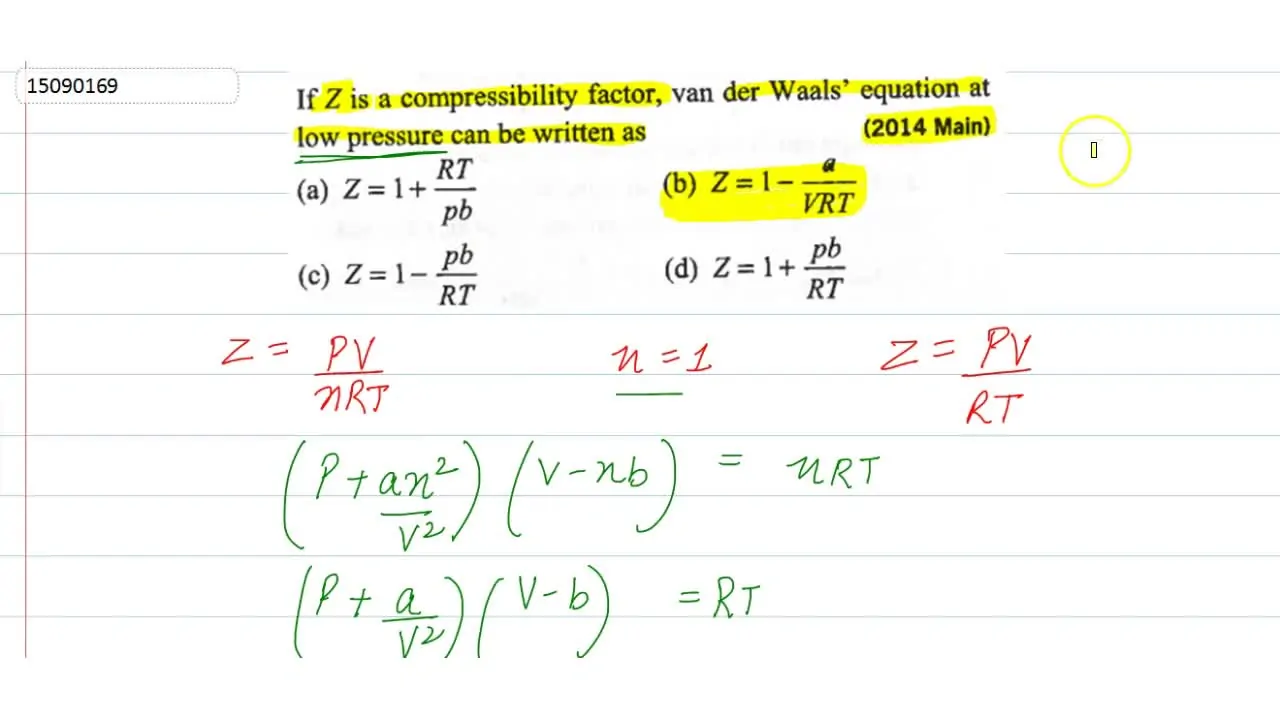
If Z is a compressibility factor, van der Waals' equation at low press

Compressibility factor - Wikipedia

For compressibility factor, Z, which of the following is /are correct?

Calculation of the Compressibility Factor z for Gases Using the Redlich-Kwong Equation of State with an Example for Propane, PDF, Mathematical Physics

Calculation of the Compressibility Factor z for Gases Using the Redlich-Kwong Equation of State with an Example for Propane, PDF, Mathematical Physics
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as

Compressibility factor - Wikipedia

gas laws - How to find the temperature relationship between the isotherms in a compressibility factor (Z) vs pressure graph? - Chemistry Stack Exchange

Real gas z-factor, as attributed to Standing and Katz, 9 plotted as a
Solved] Solution Thermodynamics oblem 4. Examination of whether a gas
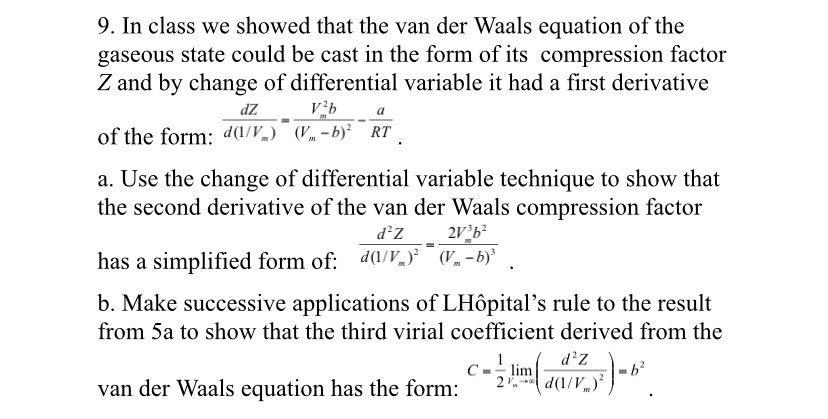
Solved 9. In class we showed that the van der Waals equation






