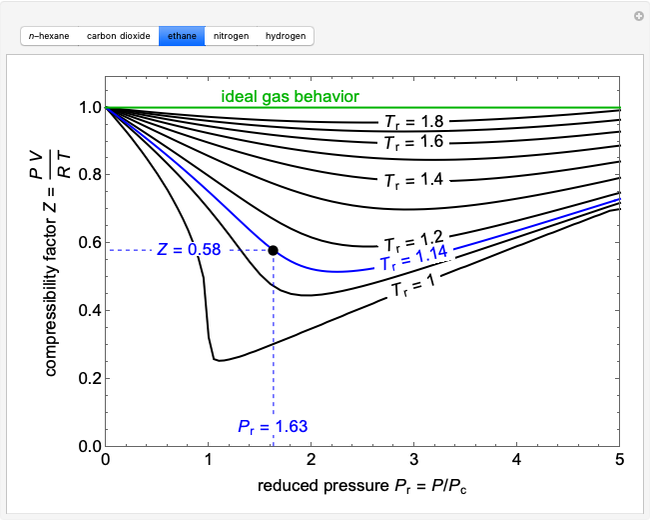
What is the value of compressibility factor in terms of vander
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
What is the value of compressibility factor in terms of vander waal cons-an t at different conditions of pressure and volume-Why is Z-1 for H2 and He gas

SOLVED: The fugacity of a van der Waals gas can be determined using the expression for the compressibility factor Z. The expression for fugacity of a van der Waals gas is given

A : At high pressure , the compressibility factor Z is (1 + (pb)/(RT))

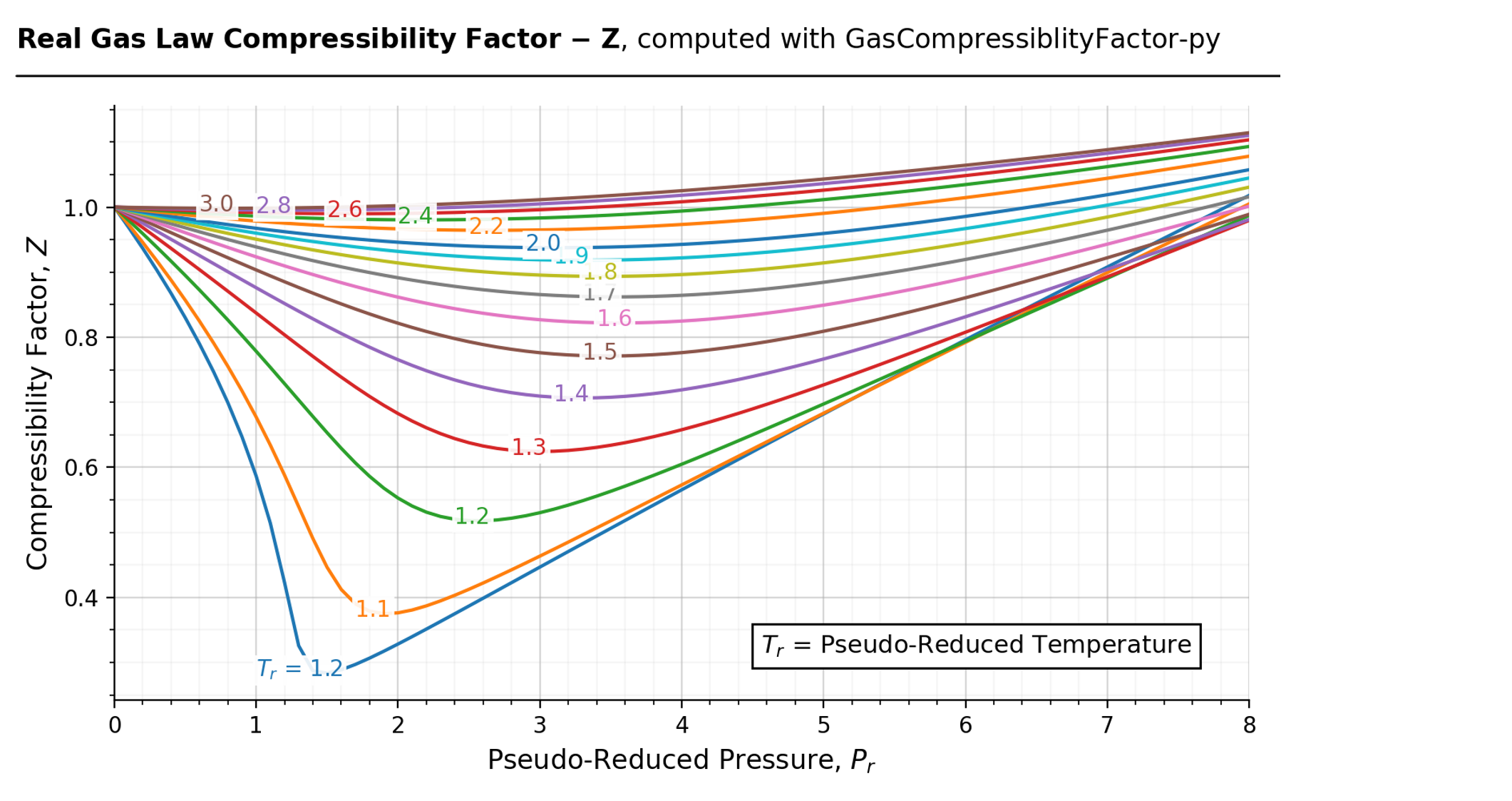
Compressibility factors of air under the specified pressure and

1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts

Compressibility factor - Wikipedia

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

If Z is a compressibility factor, van der Waals equation at low pressure ..
Slope of graph of compressibility factor(Z) with pressure(P) for hydrogen gas at any pressure i

Compressibility factor Z - Gaseous State
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas