Solved What is the equilibrium constant (Kp) at 45 °C for

Answer to Solved What is the equilibrium constant (Kp) at 45 °C for

16.6h Using the general properties of equilibrium constants
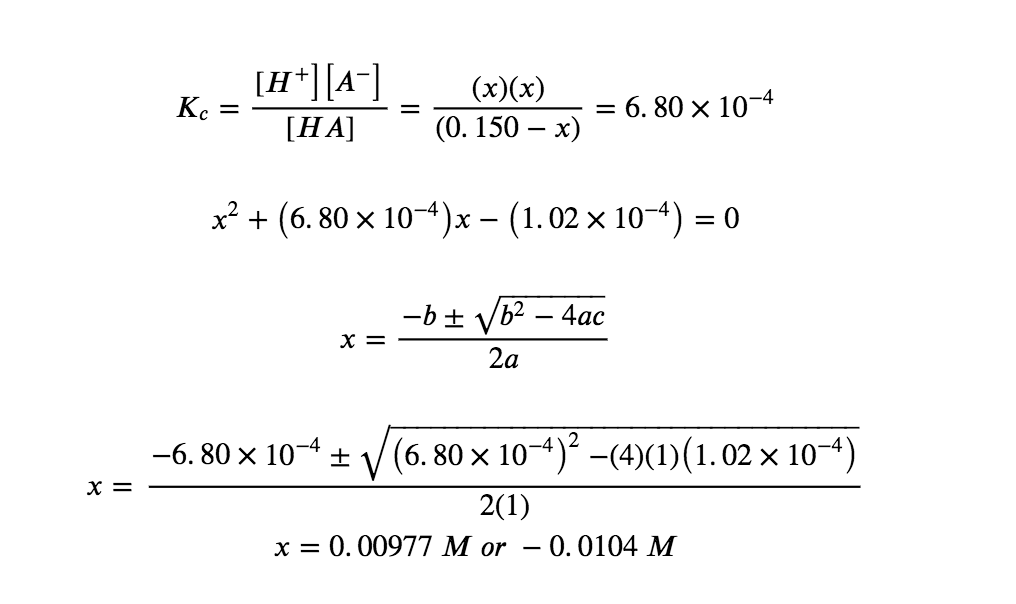
4.3 – Solving Equilibrium Problems – General Chemistry for Gee-Gees
A well structured lesson including starter activity, AfL work tasks, main work tasks with answers on The Equilibrium Constant KpBy the end of the
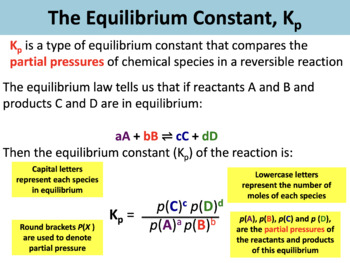
The Equilibrium Constant Kp
✓ Solved: At 2200^∘ C, Kp=0.050for the reaction N2(g)+O2(g) ⇌ 2 NO(g) What is the partial pressure of

Solved 11. For the reaction the equilibrium constant, Kp -45

16.41a Calculate the equilibrium constant at 25 °C for O2(g) + 2F2(g) → 2OF2(g) ΔG° = −9.2 kJ
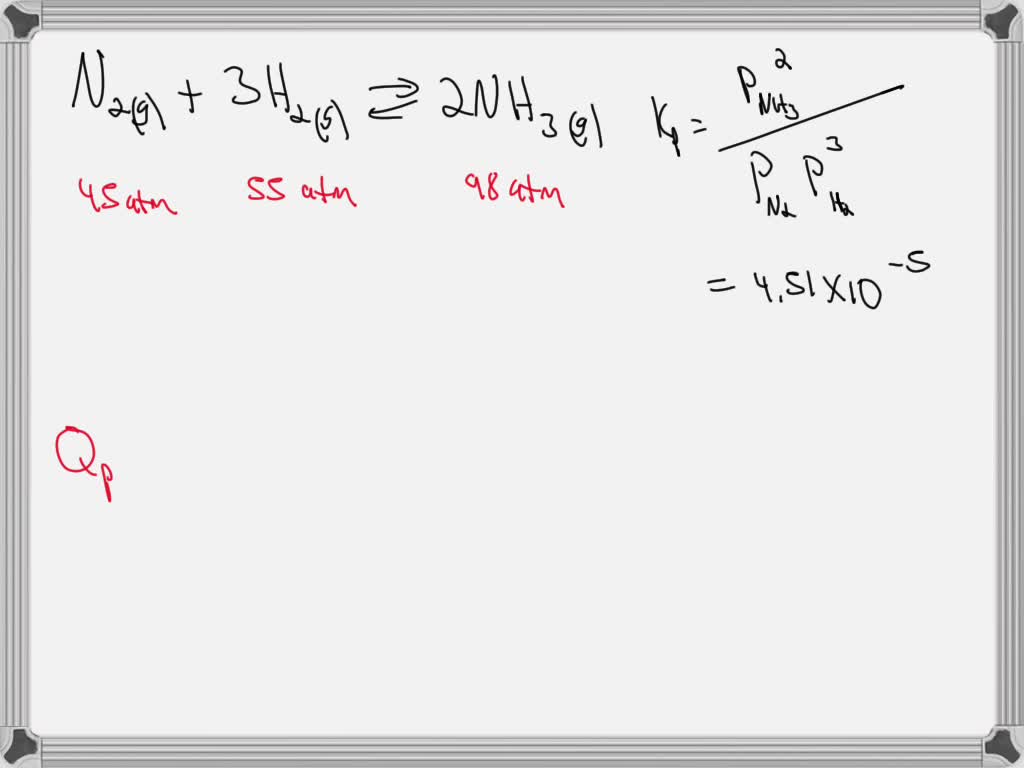
SOLVED: Kp for the equilibrium N2(g) + 3H2(g) ⇌ 2NH3(g) is 4.51 × 10-5 at 450°C. A mixture at this temperature has 98 atm NH3, 45 atm N2, and 55 atm H2.

Consider the reaction: A(g) ⇌ B(g) + C(g) Find the equilibrium co
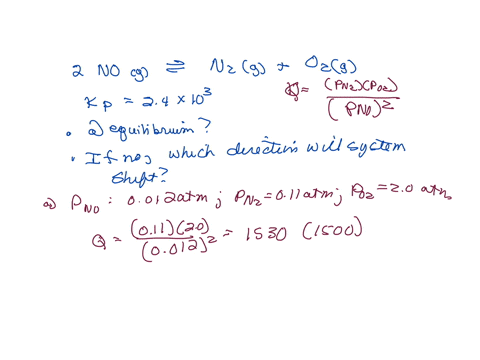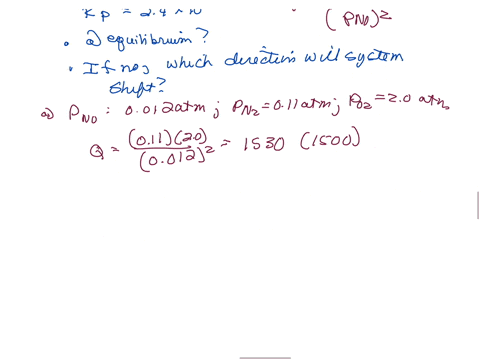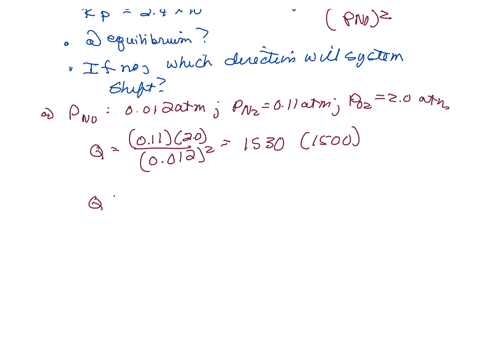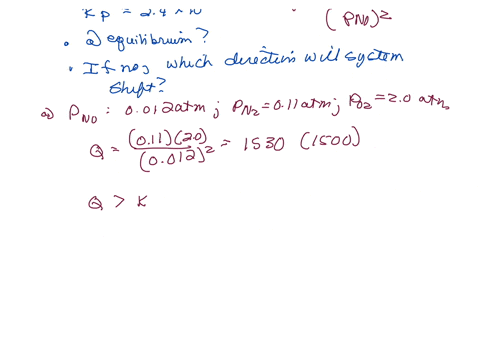
⏩SOLVED:The equilibrium constant Kp is 2.4 ×10^3 at a certain…
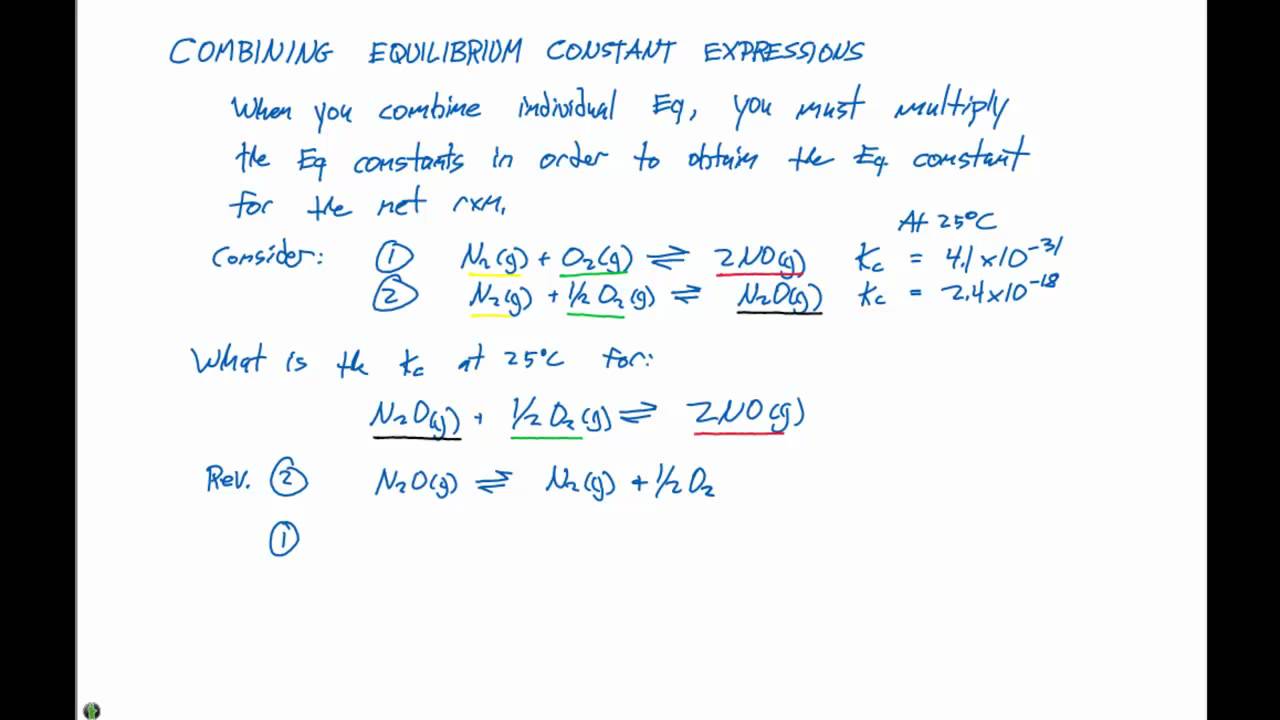
15.3 Combining Equilibrium Constants
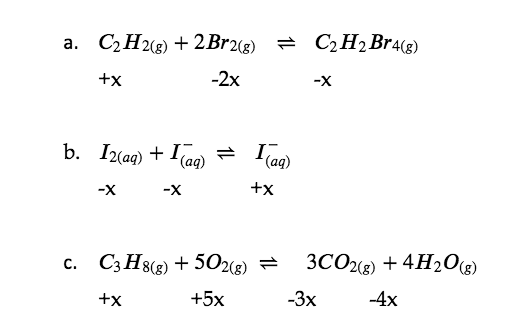
4.3 – Solving Equilibrium Problems – General Chemistry for Gee-Gees

The equilibrium constant (KP) of the reactions N2O4hArr 2NO2 was found
The equilibrium constant (K) for the reaction,2SO2(g)+O2(g)2S03(g) at 1000 K is 3.5 atmWhat would be the partial pressure of oxygen gas,if the equilibrium is found to have equal moles ofSO2 and SO3?

⏩SOLVED:At 1000 K, a sample of pure NO2 gas decomposes: 2 NO2(g) ⇌2…







