What is the shape of the carbonate ion, (CO3)^2 ?


How is VSEPR theory used to predict molecular structure? - ppt video online download
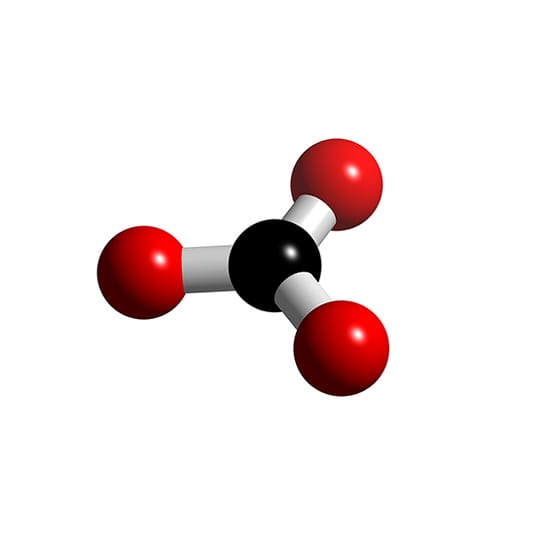
CO3]2- - Carbonate
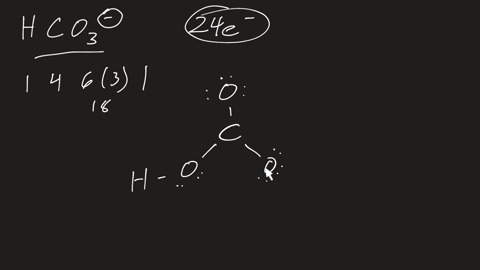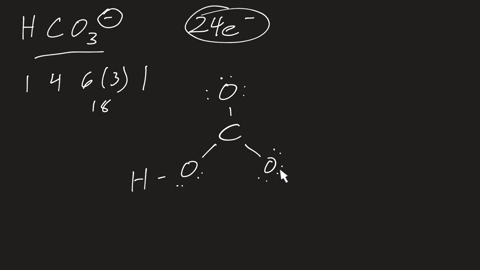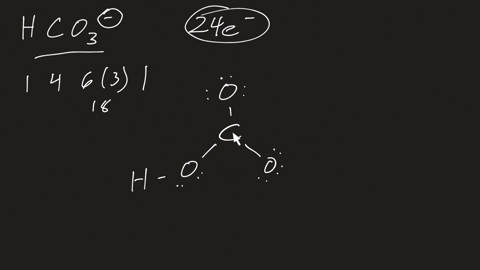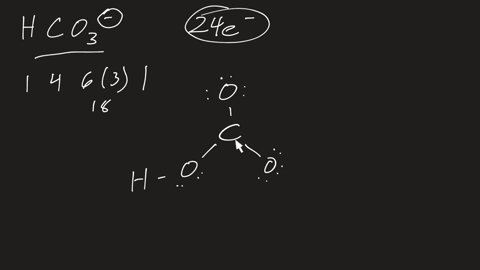
⏩SOLVED:a. Draw the resonance structures for carbonate (CO3^2-) . b.…

Determine the molecular geometry of the carbonate ion, CO32-. a. bent b. trigonal planar c. trigonal bipyramidal d. tetrahedral e. trigonal pyramidal

CO32- Molecular Geometry, Shape and Bond Angles (Carbonate Ion)
Draw a Lewis structure for CO32- and answer the following questions. a. What is the number of lone pairs? b. What is the number of single bonds? c. What is the number

SOLVED: Carbonate and Bicarbonate 1. Determine the following for both ions: a. Lewis structure (all 3 resonant structures!) b. Electronic geometry (about the carbon atom) c. Molecular geometry (about the carbon atom)

Draw the structure of CO32-. Include all lone pairs of electrons and formal charges. Draw the ion by placing atoms on the grid and connecting them with bonds. Include all lone pairs
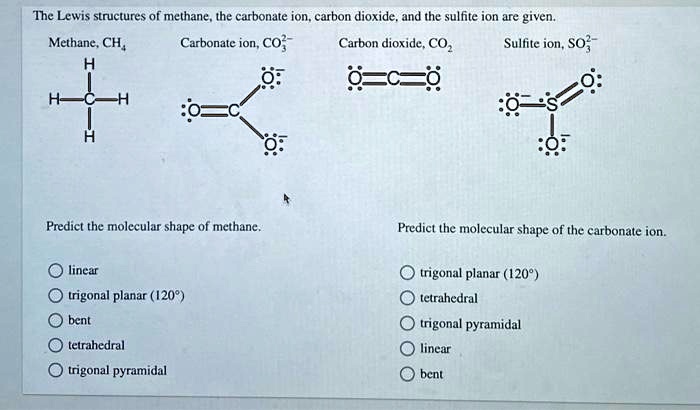
SOLVED: The Lewis structures of methane, the carbonate ion, carbon dioxide, and the sulfite ion are given. Methane (CH4), Carbonate ion (CO3^2-), Carbon dioxide (CO2), and Sulfite ion (SO3^2-) are represented. Predict

carbonate ion is CO3 2- No . of hybrid orbitals ,.pdf
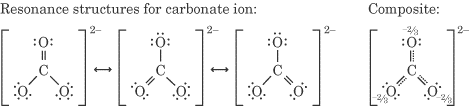
How do resonance structures affect molecular shape?

Is CO32- Polar or Nonpolar? - Polarity of Carbonate ion

Carbon trioxide - Wikipedia
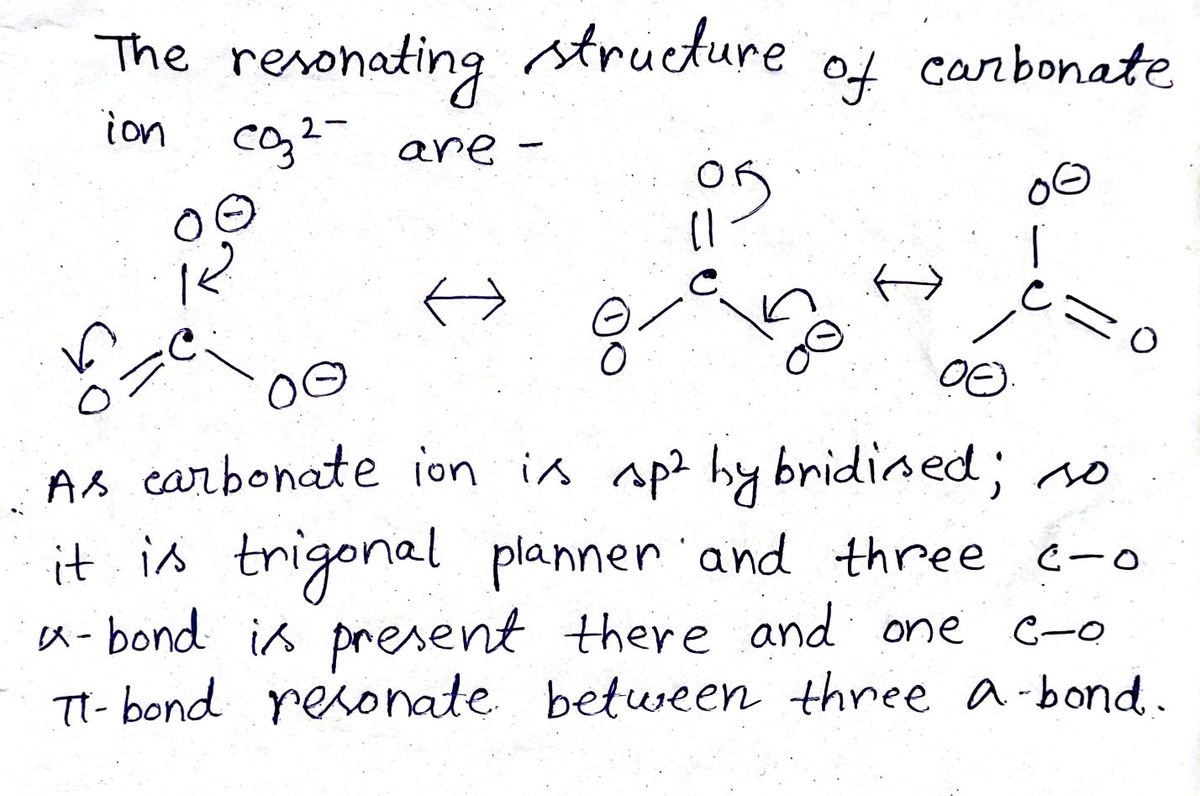
Answered: carbonate ion, CO32−,CO32−, draw all of…
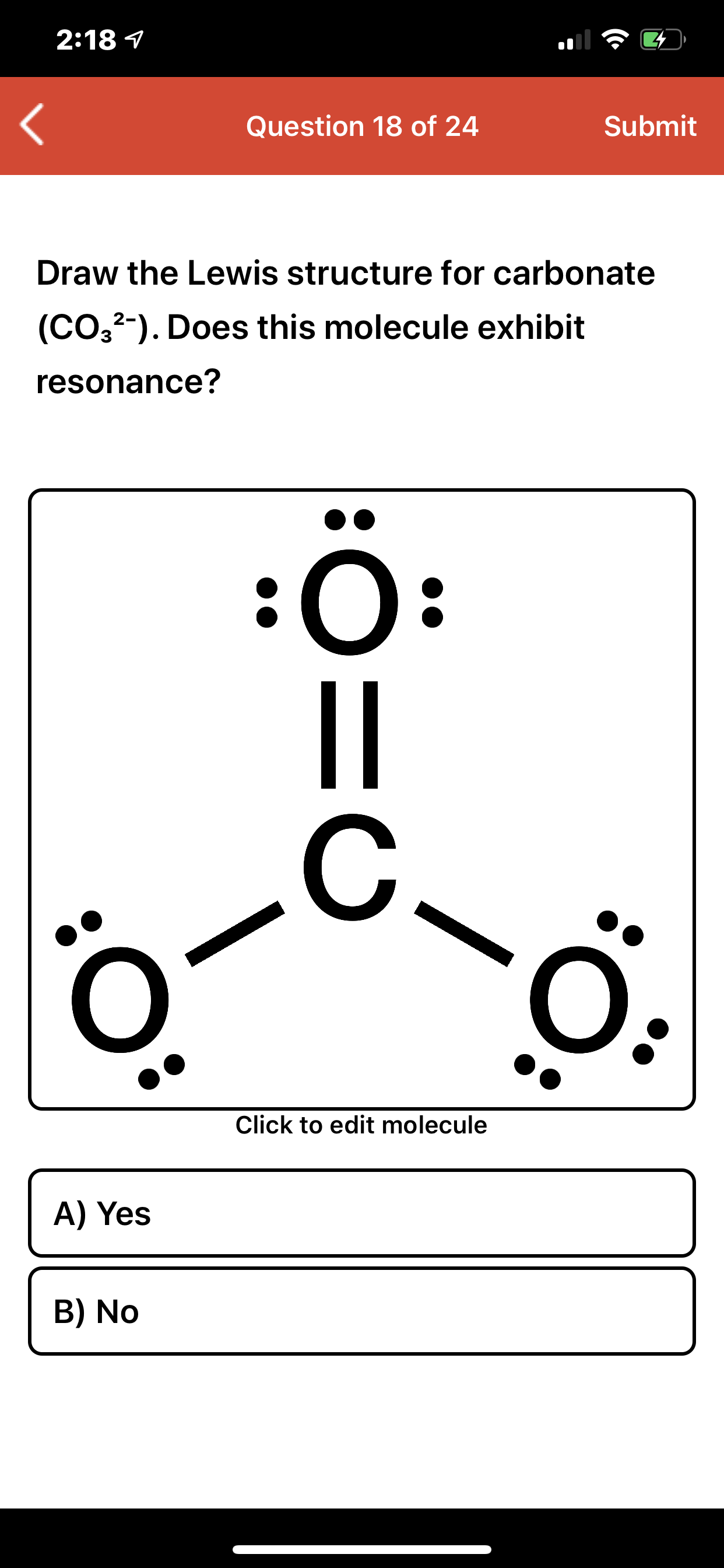
Answered: 2:18 1 Question 18 of 24 Submit Draw…