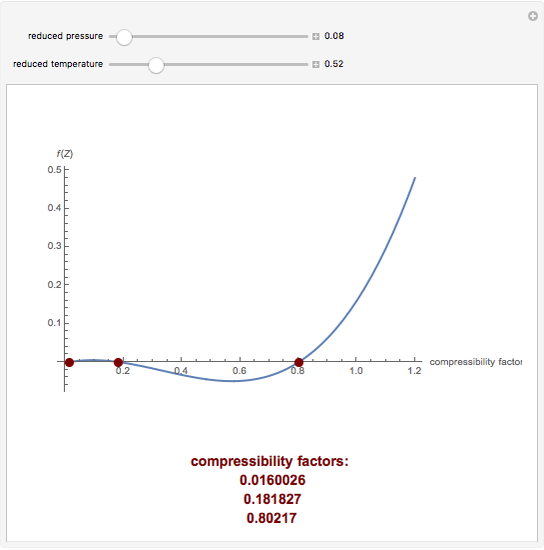
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
What is the value of compressibility factor in terms of vander waal cons-an t at different conditions of pressure and volume-Why is Z-1 for H2 and He gas

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

A : At high pressure , the compressibility factor Z is (1 + (pb)/(RT))

Solved We begin by showing that the compressibility factor

Compressibility Factor - an overview
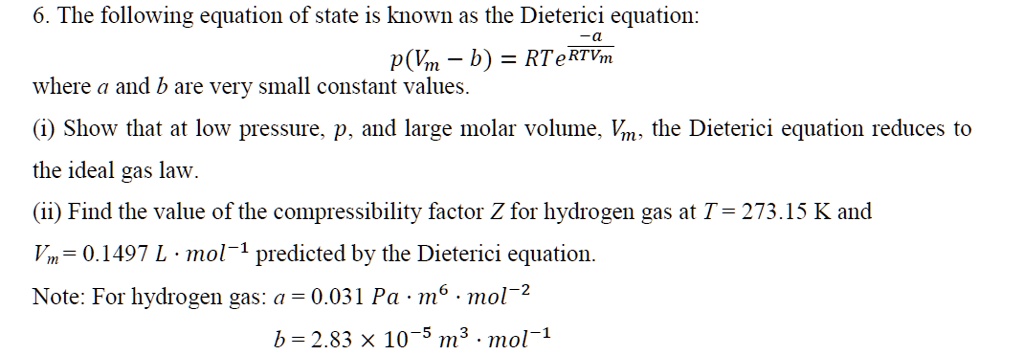
SOLVED: The following equation of state is known as the Dieterici equation: p(Vm - b) = RT * e^(RT/Vm), where a and b are very small constant values. Show that at low
How I find the a and b constant in the Van der Waals equation? - Quora
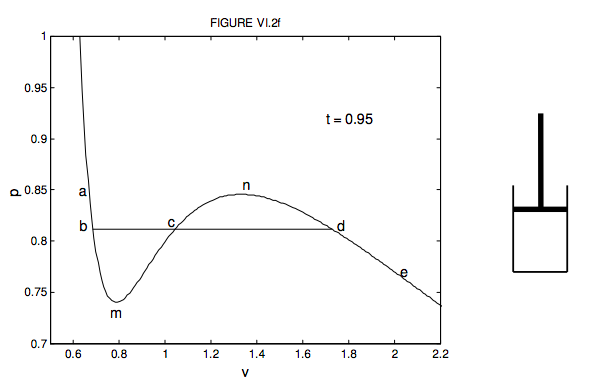
6.3: Van der Waals and Other Gases - Physics LibreTexts

What is the compressibility factor (Z) for 0.02 mole of a van der Waal
What characteristics describe ideal gases? - Quora