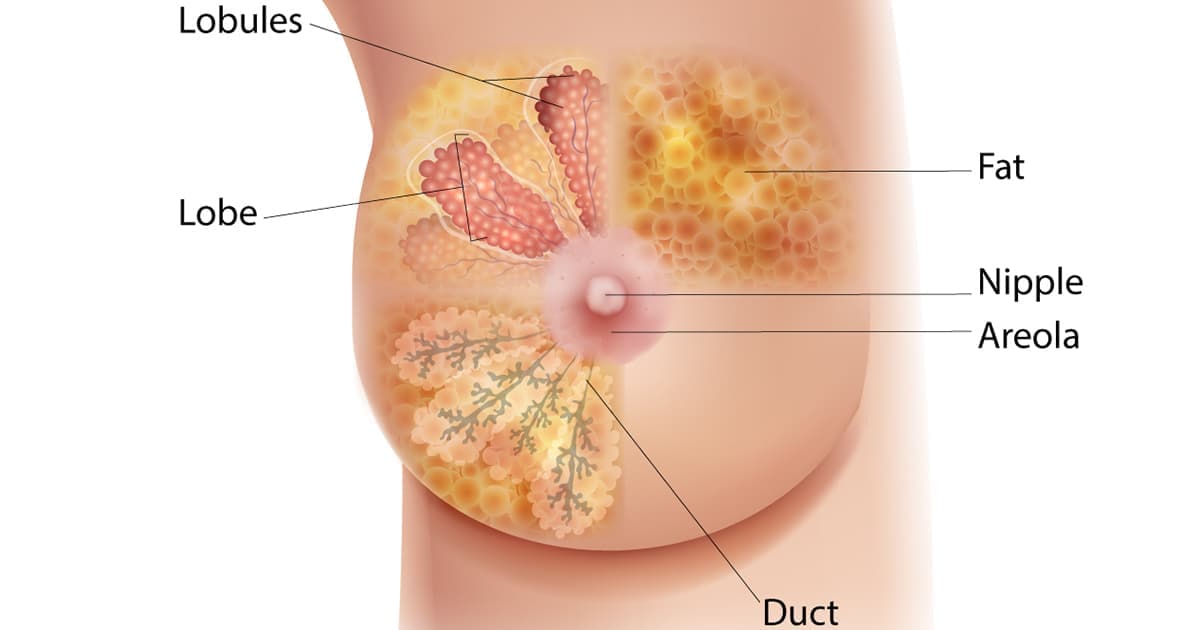
FDA has new mammogram guidelines for dense breast disclosure. What do the rules mean for Pennsylvania residents?
/cloudfront-us-east-1.images.arcpublishing.com/pmn/EMEDJI4AQVBZJNJHKLTY2ZSPJY.jpg)
In Pennsylvania, senators unanimously voted in favor of a bill to fund genetic testing to women at higher risk of breast cancer.

FDA sets new guidelines that require mammogram providers to notify about breast density

The FDA's rule change requiring providers to inform women about breast density could lead to a flurry of questions
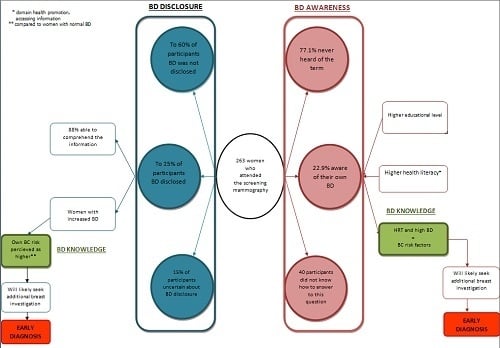
Diagnostics, Free Full-Text
:max_bytes(150000):strip_icc():focal(999x625:1001x627)/breast-cancer-1-7c0d710af45749f2baec134af348d778.jpg)
FDA to Implement New Mammogram Regulations to Support Women with Dense Breasts

Medical practitioners will have to notify patients about breast density in mammograms under new FDA regulations - CBS News

Rad Tech CE, ASRT, ARRT® CE, Category A Credits

FDA mammogram guidelines: People with dense breasts to be notified

The FDA's rule change requiring providers to inform women about breast density could lead to a flurry of questions - UPMC & Pitt Health Sciences News Blog

Vision 20/20: Mammographic breast density and its clinical applications - Ng - 2015 - Medical Physics - Wiley Online Library