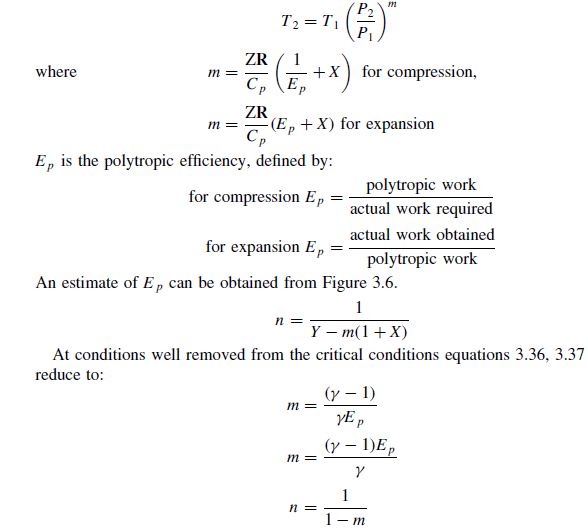
At 273 K measurements on argon gave B = -21.7 cm$^3$ mol$^{


What volume is occupied by 12.5 g of argon gas at a pressure of 1

Effective recombination rate coefficients αeff−ion > at different H2 >

SOLVED: At 273 K, measurements on argon gave B = -21.7 cm^3/mol and C = 1200 cm^6/mol^2, where B and C are the second and third virial coefficients in the expression of

Solved 6. Take the van der Waals equation RT a 2 a. Express

Magnetochemistry, Free Full-Text

Raw images observed at mass 56, with a discharge in argon ͑ a ͒ or

Left) Argon adsorption ( fi lled symbols) and desorption (open
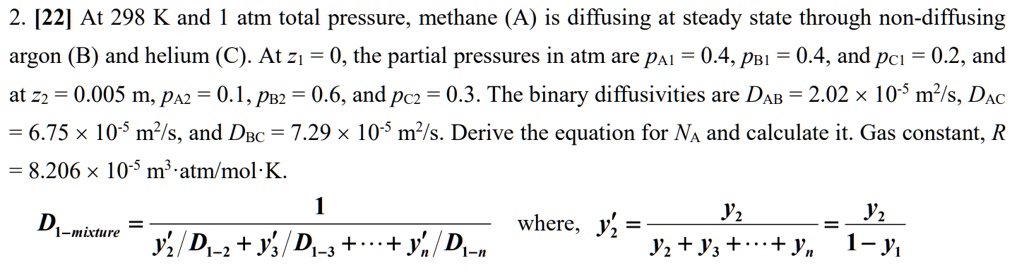
SOLVED: At 298 K and 1 atm total pressure, methane (A) is diffusing at steady state through non-diffusing argon (B) and helium (C). At z1 = 0, the partial pressures in atm

Chap1
Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law
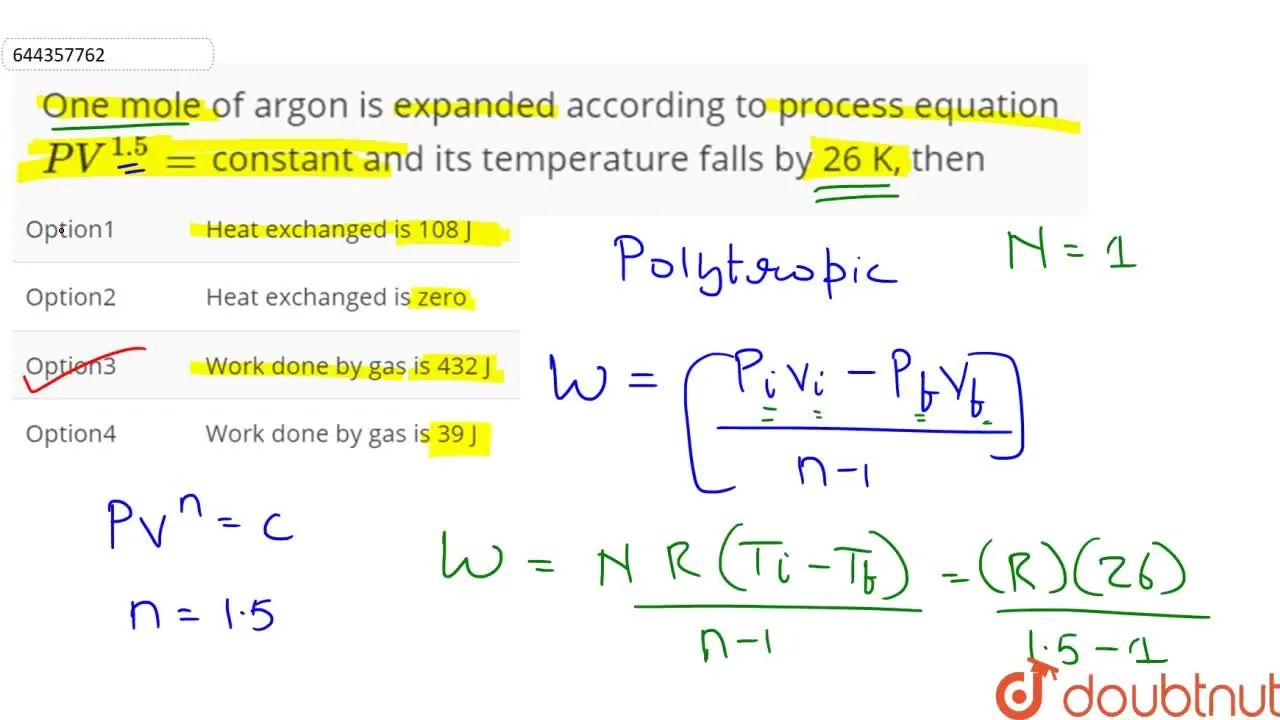
One mole of argon is expanded according to process equation PV^(1.5)=c
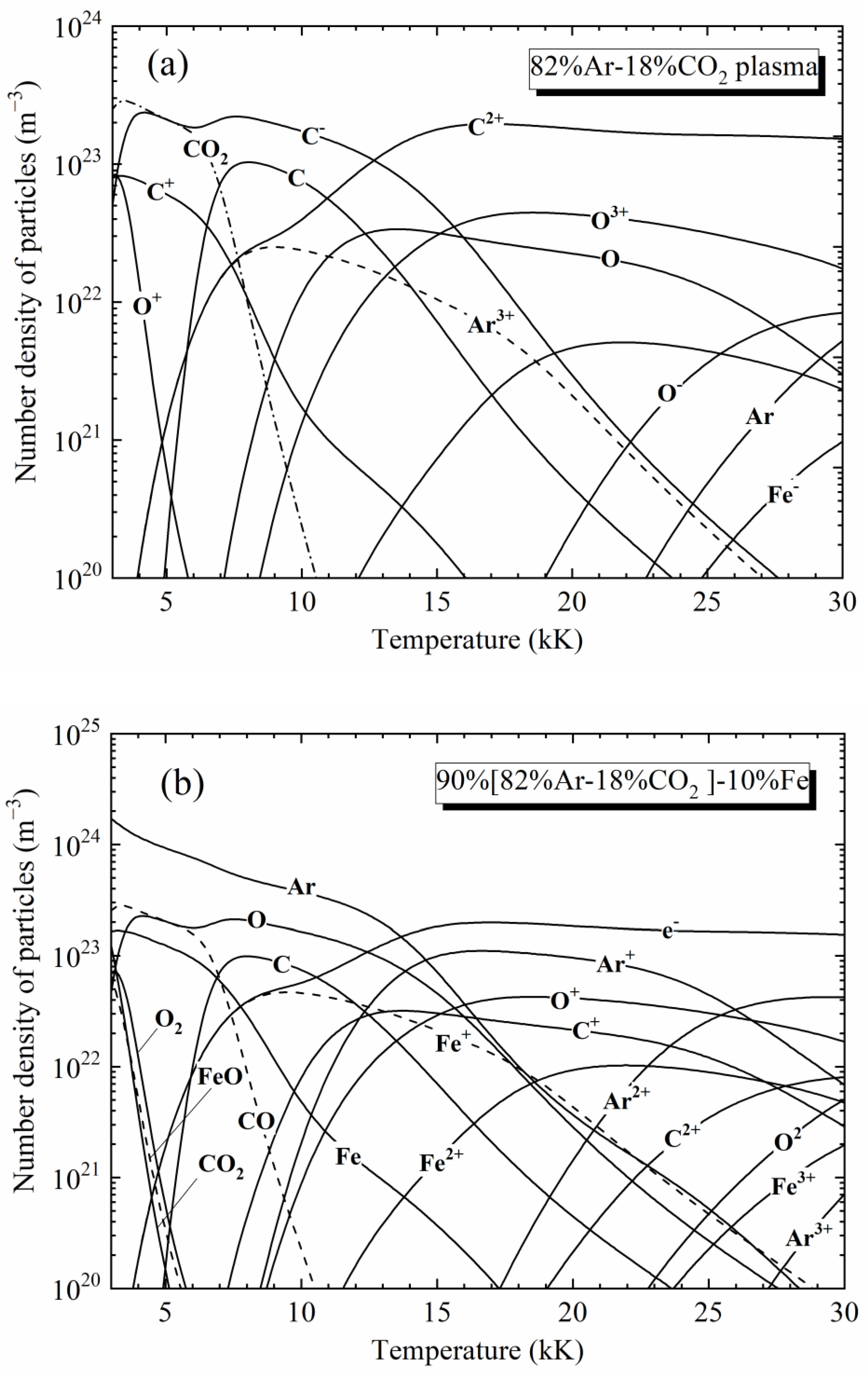
Materials, Free Full-Text