At high pressure, the compressibility factor 'Z' is equal toa)unityb) c) d)ZeroCorrect answer is option 'C'. Can you explain this answer? - EduRev NEET Question


Except H(2) and He, the compressibility factor Z(=(PV)/(nRT))lt1 for a
If Z is a compressibility factor, van der Waals' equation at low pressure can be written as - Sarthaks eConnect

Solved Shown below are compressibility data for nitrogen

The compressibility factor a real gas high pressure is:1+ dfrac{RT}{pb}1+ dfrac{pb}{RT}11- dfrac{pb}{RT}

1. The compressibility factor, z, is the ratio of

If Assertion is true statement but Reason is false, then mark (3)

What is the compressibility factor (Z) for 0.02 mole of a van der Waal
Gaseous State Questions for JEE exam - Free Online All questions of Gaseous State - Chapter-wise Questions of JEE

Van der Waals Equation (Old NCERT) Free MCQ Practice Test with Solutions - NEET
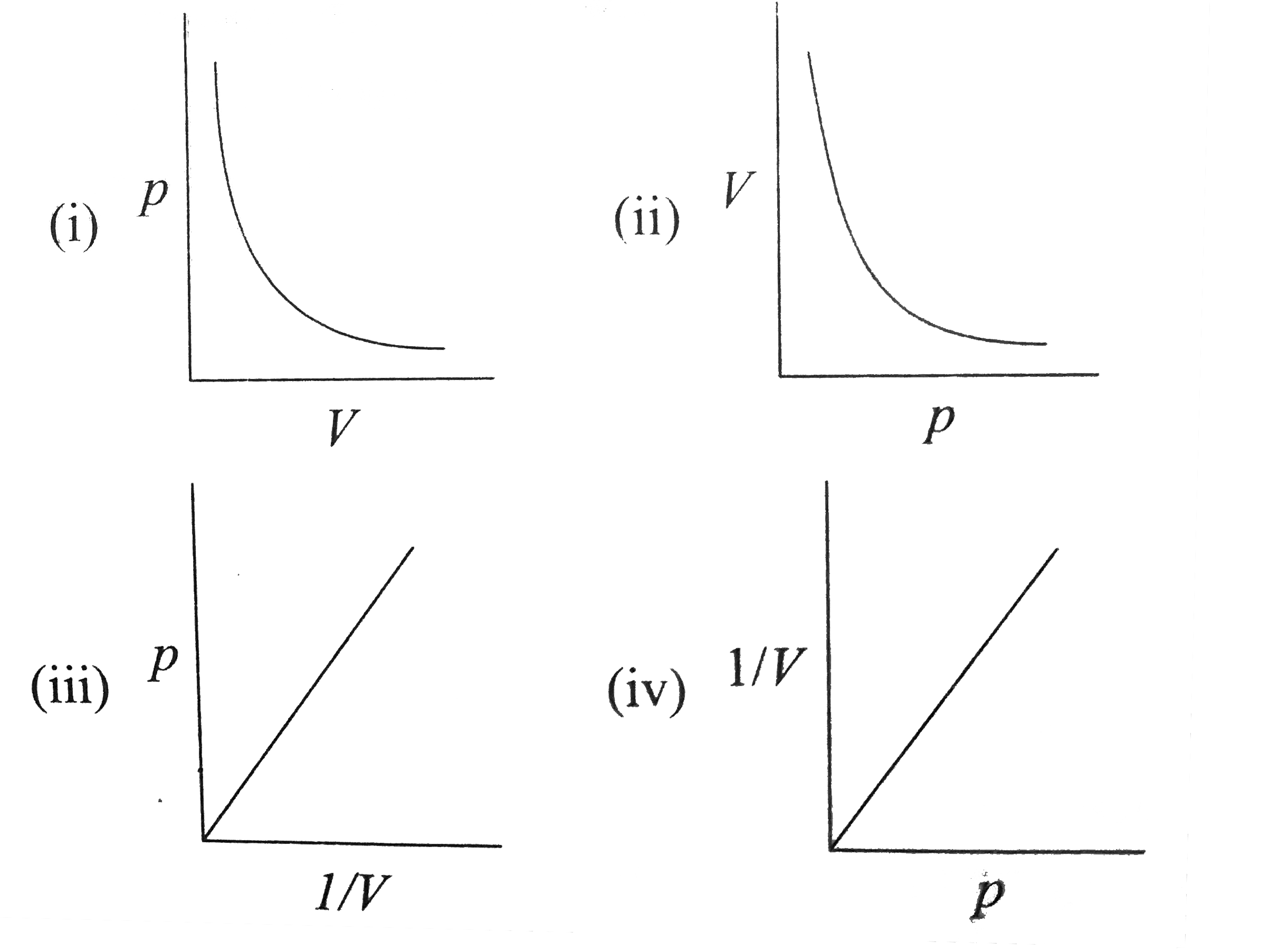
If assertion is true but reason is false.

For compressibility factor, Z, which of the following is /are correct?
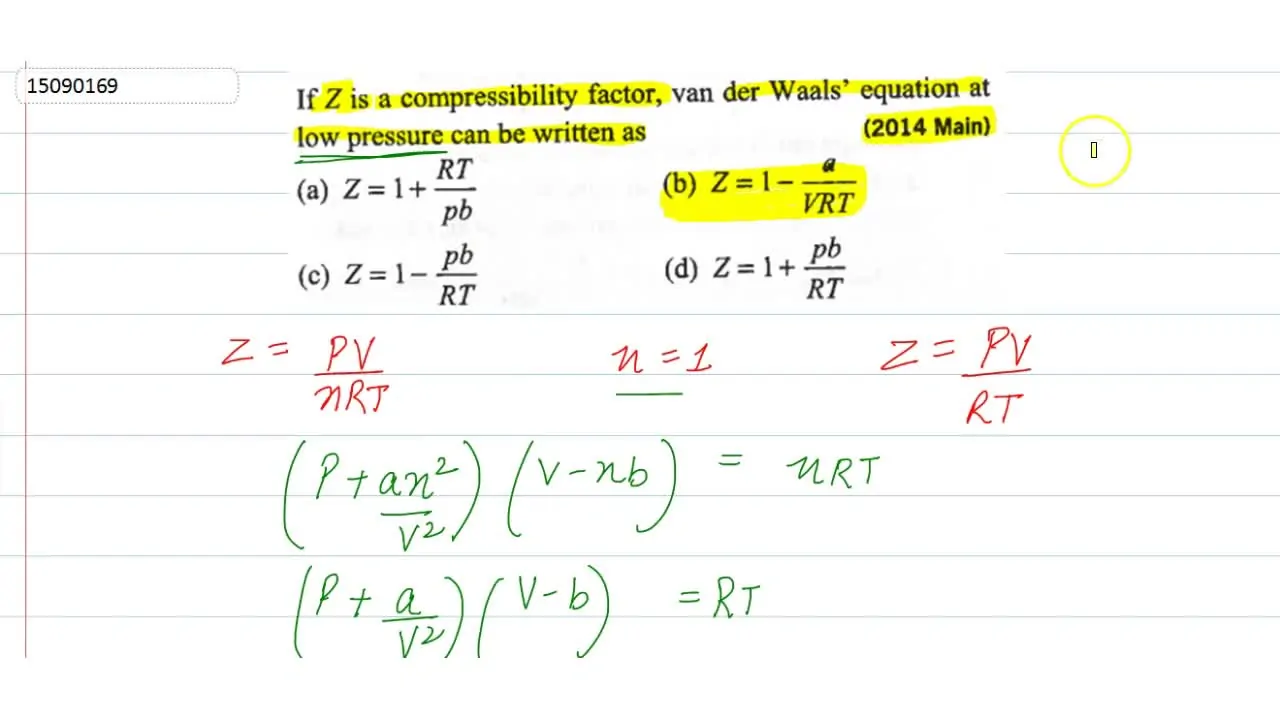
If Z is a compressibility factor, van der Waals' equation at low press
Van der Waals Equation (Old NCERT) Free MCQ Practice Test with Solutions - NEET

At a high pressure, the compressibility factor (Z) of a real gas is us