1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts
By A Mystery Man Writer

Why is the volume of gas particles assumed to be negligible by the Kinetic Molecular Theory of Gases? - Quora
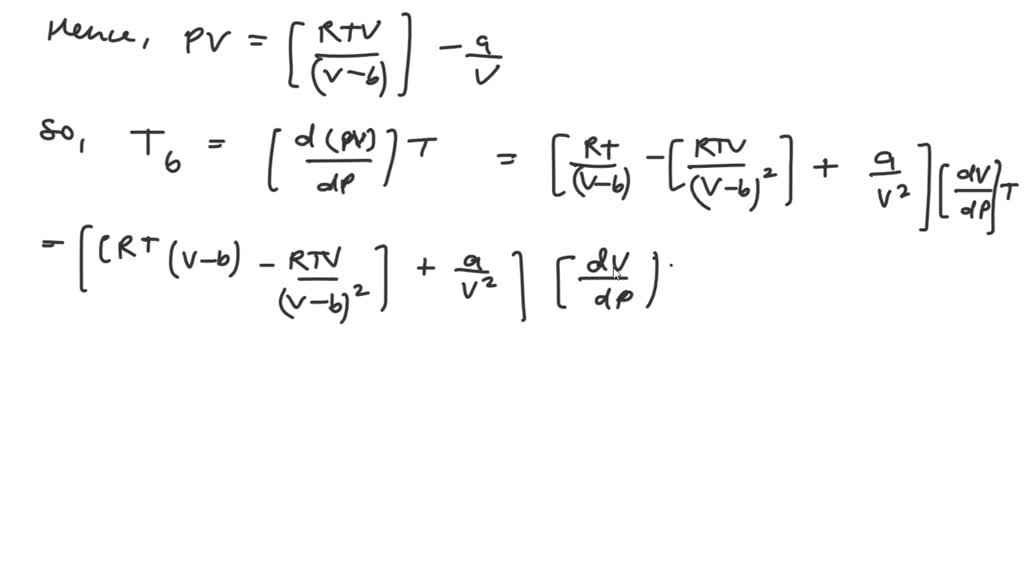
SOLVED: Find the mathematical expression for the Boyle temperature of a van der Waals gas, then find the value of the Boyle temperature of chlorine gas as predicted by the van der
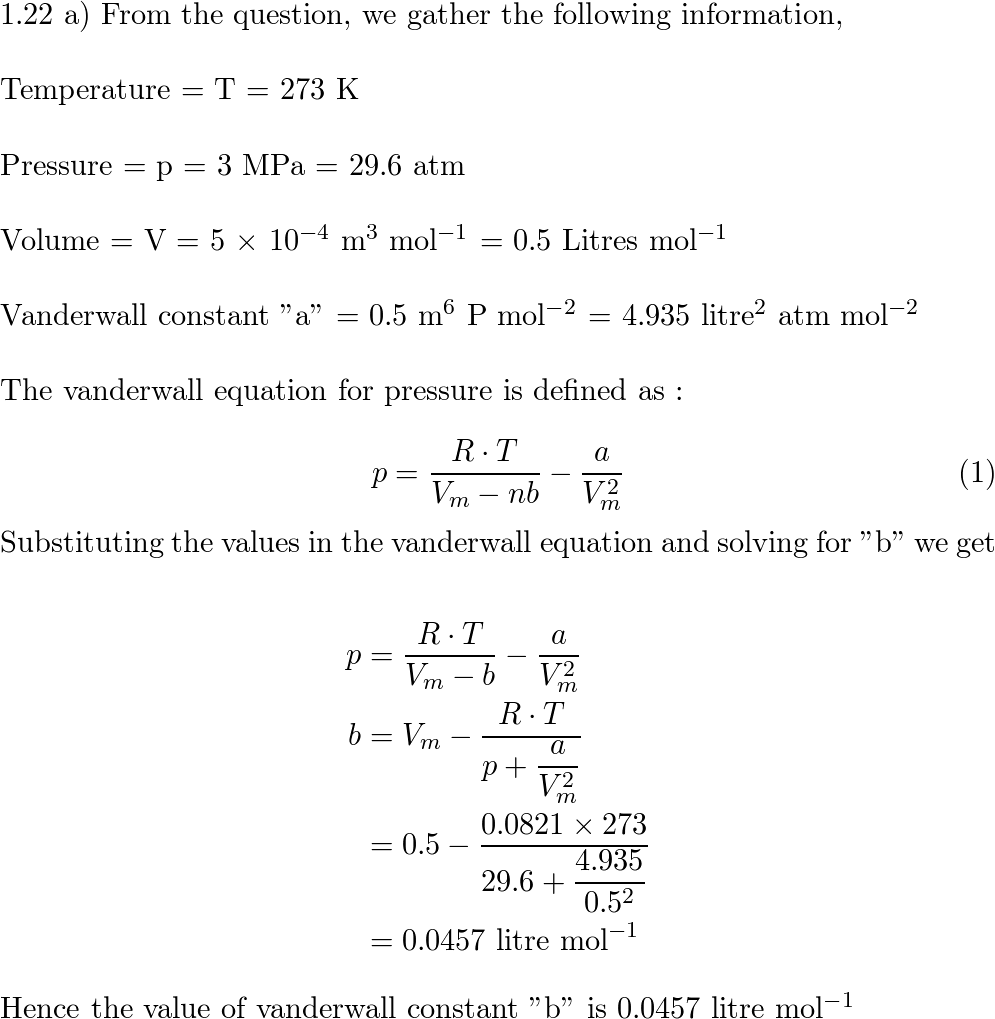
a) A certain gas obeys the van der Waals equation with $a =
UAH Global Temperature Update for February, 2023: +0.08 deg. C « Roy Spencer, PhD

Van der Waals Equation Practice Problems

Van der Waals Equation Practice Problems
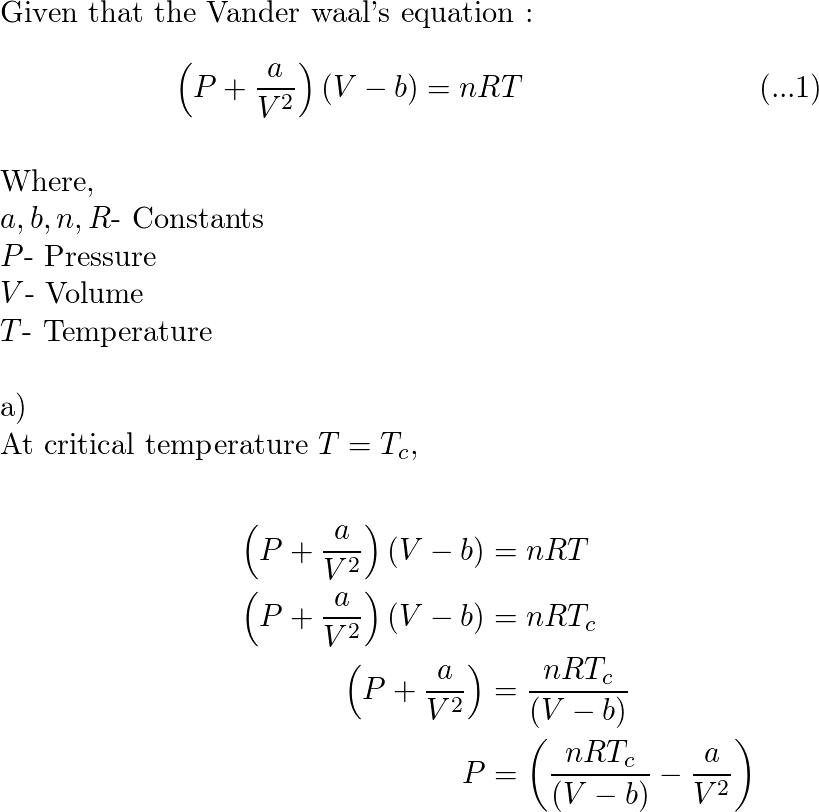
In physical chemistry, it is shown that the pressure P of a

Full PDF, PDF, Cell Nucleus
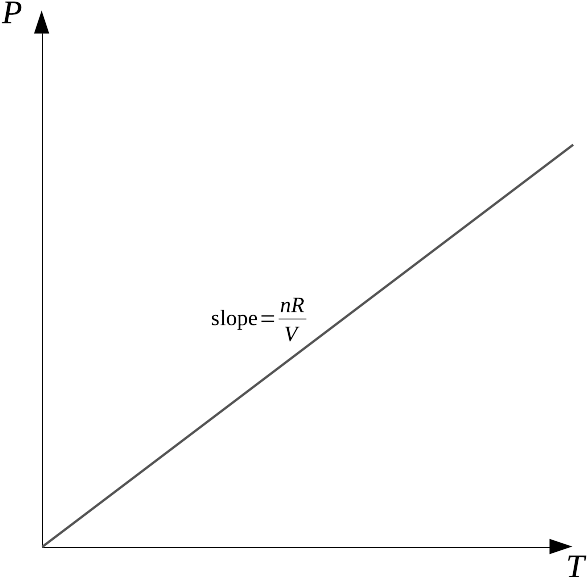
1.8: The ideal gas law, functions and derivatives - Chemistry LibreTexts







