How to Calculate Normality of a Solution
:max_bytes(150000):strip_icc()/how-to-calculate-normality-609580final2-0d5efa5a961f4fa0a7efc780921faee1.png)
The normality of a solution is the gram equivalent weight of a solute per liter of solution. Here are examples of how to calculate the normality.
calculate the normality of250 ml aqueous solution ofH2SO4 having pH=0.0
Solved] This is a Normality Problem that already shows the solution. I am

Calculate the volume strength of H_(2)O_(2) solution if 50 mL of H_(2)O_(2) solution is diluted

10 g impure NaOH is completely neutralised by 1000 ml of `(1)/(10)N HCl`. Calculate the pe

How to Calculate Normality: 4 Steps (with Pictures) - wikiHow
What is the normality of solution obtained by mixing 100 ml of 0.2 M H2SO4 with 100 ml of 0.2 M NaOH? - Quora

Normality calculation - example problems
Calculate the maximum normality of a solution containing 13.4 g of sodium oxalate in 100ml solution.
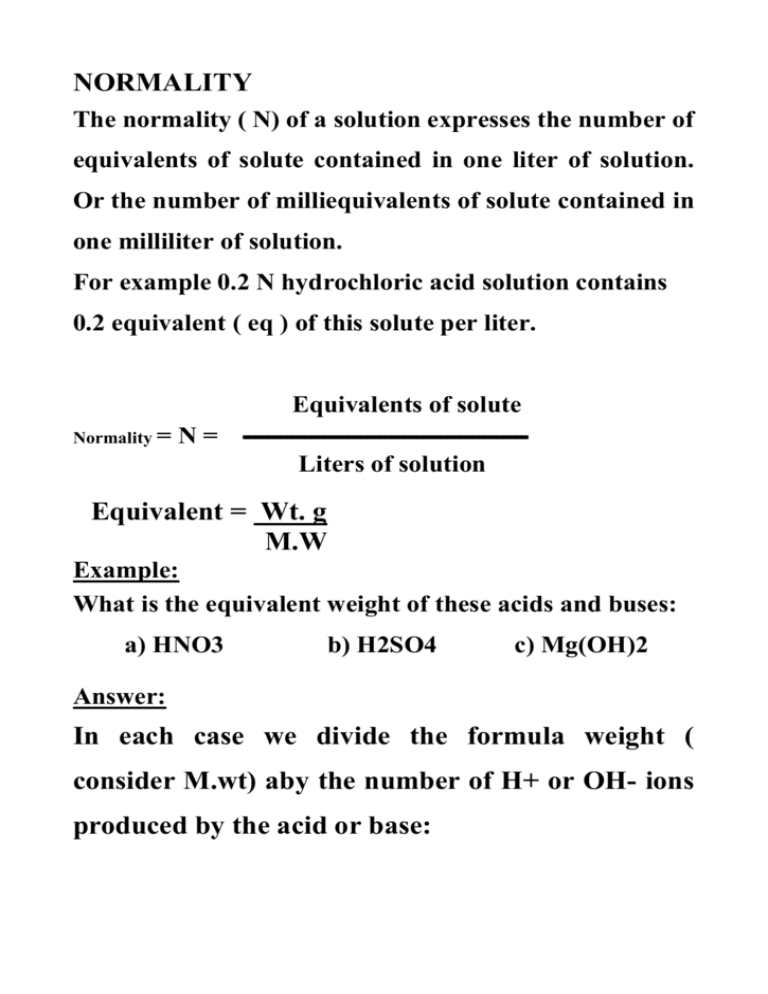
NORMALITY

Normality - Formula, Definition, Calculations [Solved Examples]

/_borders/mars-hill-maine.jpg

Determine the normality of NaOH and HCl after

SOLVED: 8. Calculate the equivalent weight and normality of a 6.0 M H3PO4 solution.
:max_bytes(150000):strip_icc()/scientist-performing-experiment-530887598-580f6db25f9b58564ccb5bcb.jpg)
How to Calculate Normality of a Solution

Define Normality and calculate the Normality of KOH solution containing 11.2g of KOH in 1 litre?







