Physical Chemistry The Compression Factor (Z) [w/1 example]

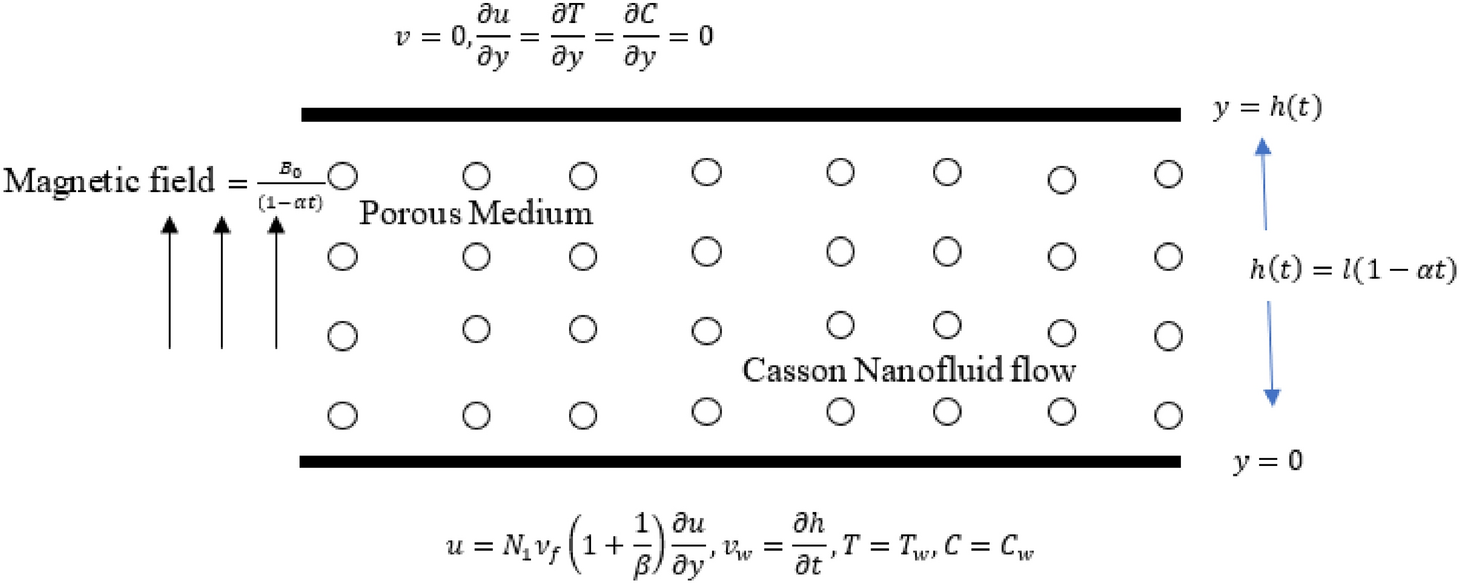
Effects of activation energy and chemical reaction on unsteady MHD dissipative Darcy–Forchheimer squeezed flow of Casson fluid over horizontal channel

The compression factor (compressibility factor) for 1 mol of a van der Waals gas at 0^(@)C and 1

The compression factor compressibility factor for 1 mole of a van der Waals' gas at 0∘ C and 100 atmospheric pressure is found to be 0.5 . Assuming that the volume of

Frontiers Smoothed particle hydrodynamics method for free surface flow based on MPI parallel computing

1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts

Determine Compressibility of Gases
Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora

Solved 1) The compression factor, Z, can be written as: Z =

Compressibility of Liquids - an overview
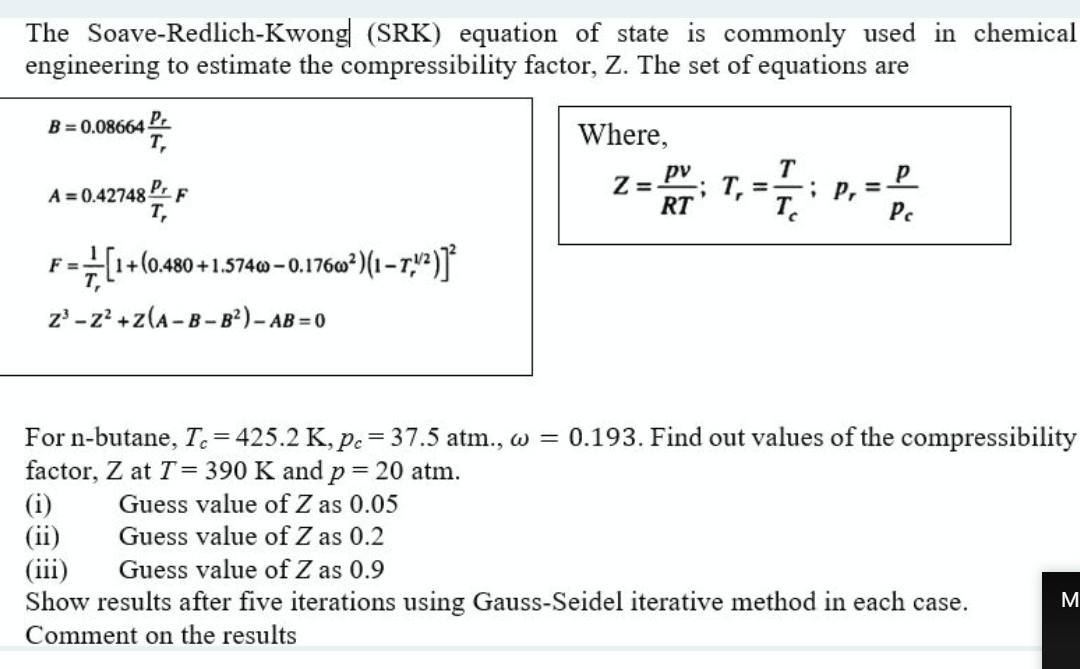
Solved The Soave-Redlich-Kwong (SRK) equation of state is

Thermodynamics Lecture 7: Compressibility

Compressibility factor (gases) - Citizendium
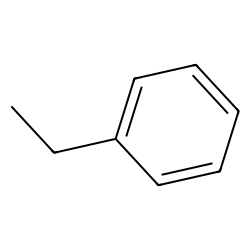
Ethylbenzene (CAS 100-41-4) - Chemical & Physical Properties by Cheméo

COMPRESSIBILITY factor Z, Using P and v in 3 Minutes!



:format(webp)/https://static-sg.zacdn.com/p/penti-2467-0667923-1.jpg)
:max_bytes(150000):strip_icc()/FortiesFashion2-2000-3a9ba55c158f4a8fb0aad4388fcf4c47.jpg)
