Aβ(1-42) tetramer and octamer structures reveal edge conductivity

The amyloid-inhibiting NCAM-PrP peptide targets Aβ peptide aggregation in membrane-mimetic environments. - Abstract - Europe PMC

Effect of lipid saturation on amyloid-beta peptide partitioning and aggregation in neuronal membranes: molecular dynamics simulations

Aβ(1-42) tetramer and octamer structures reveal edge conductivity pores as a mechanism for membrane damage
Molecules, Free Full-Text

Analyzing Morphological Properties of Early-Stage Toxic Amyloid β Oligomers by Atomic Force Microscopy
IJMS, Free Full-Text

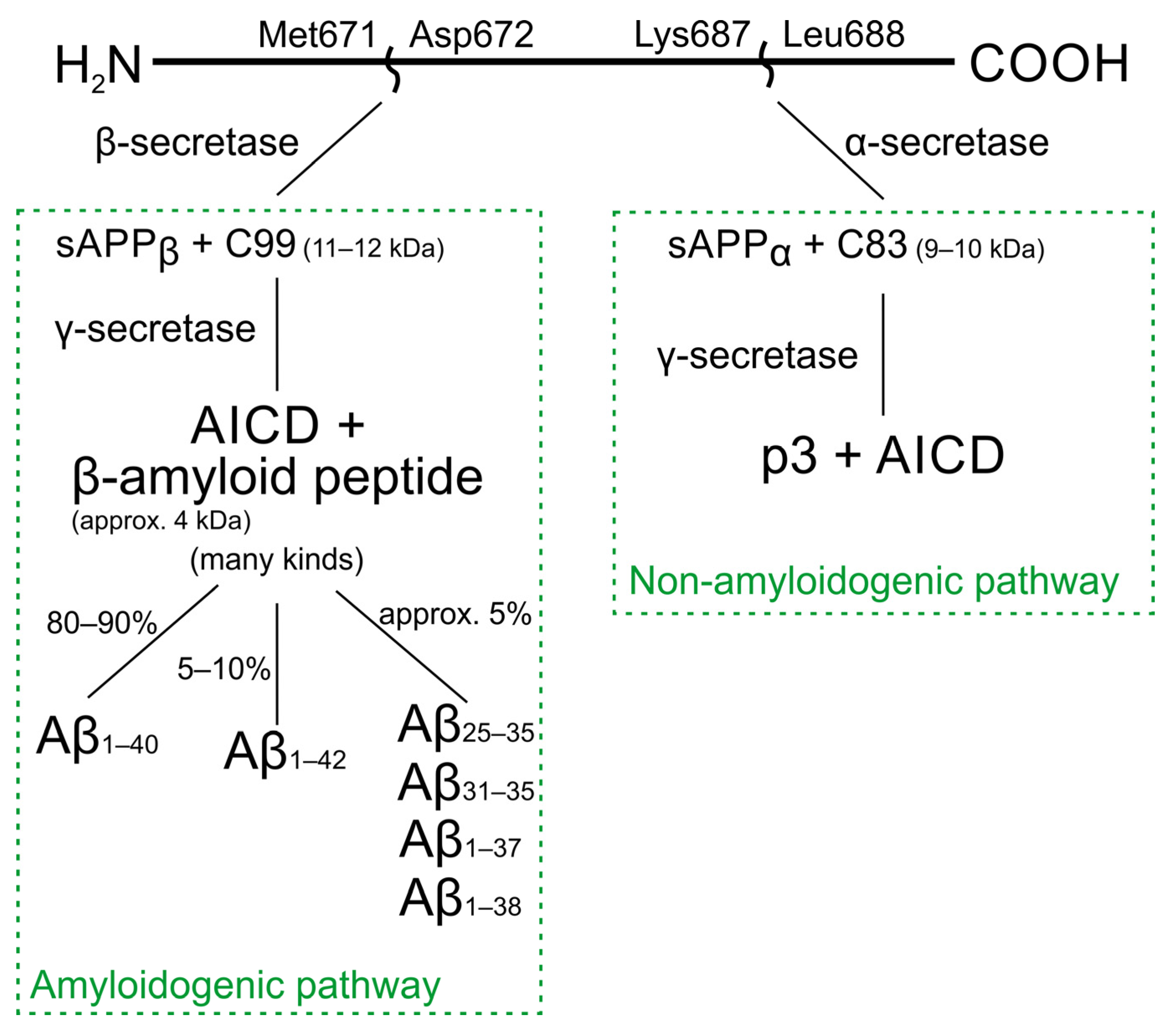
βPFOAβ(1-42) samples can be enriched in either tetramers or
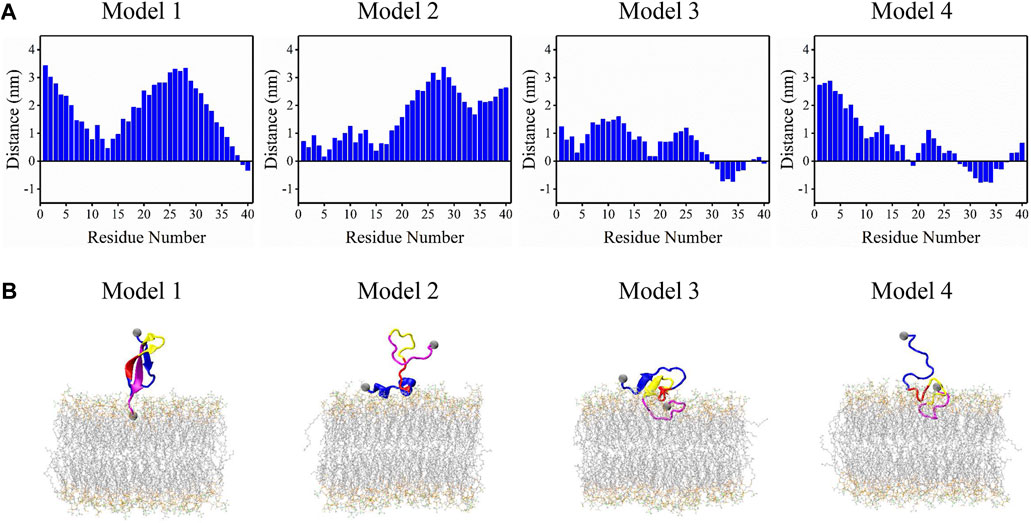
Frontiers Binding mechanism of full-length Aβ40 peptide to a mixed lipid bilayer
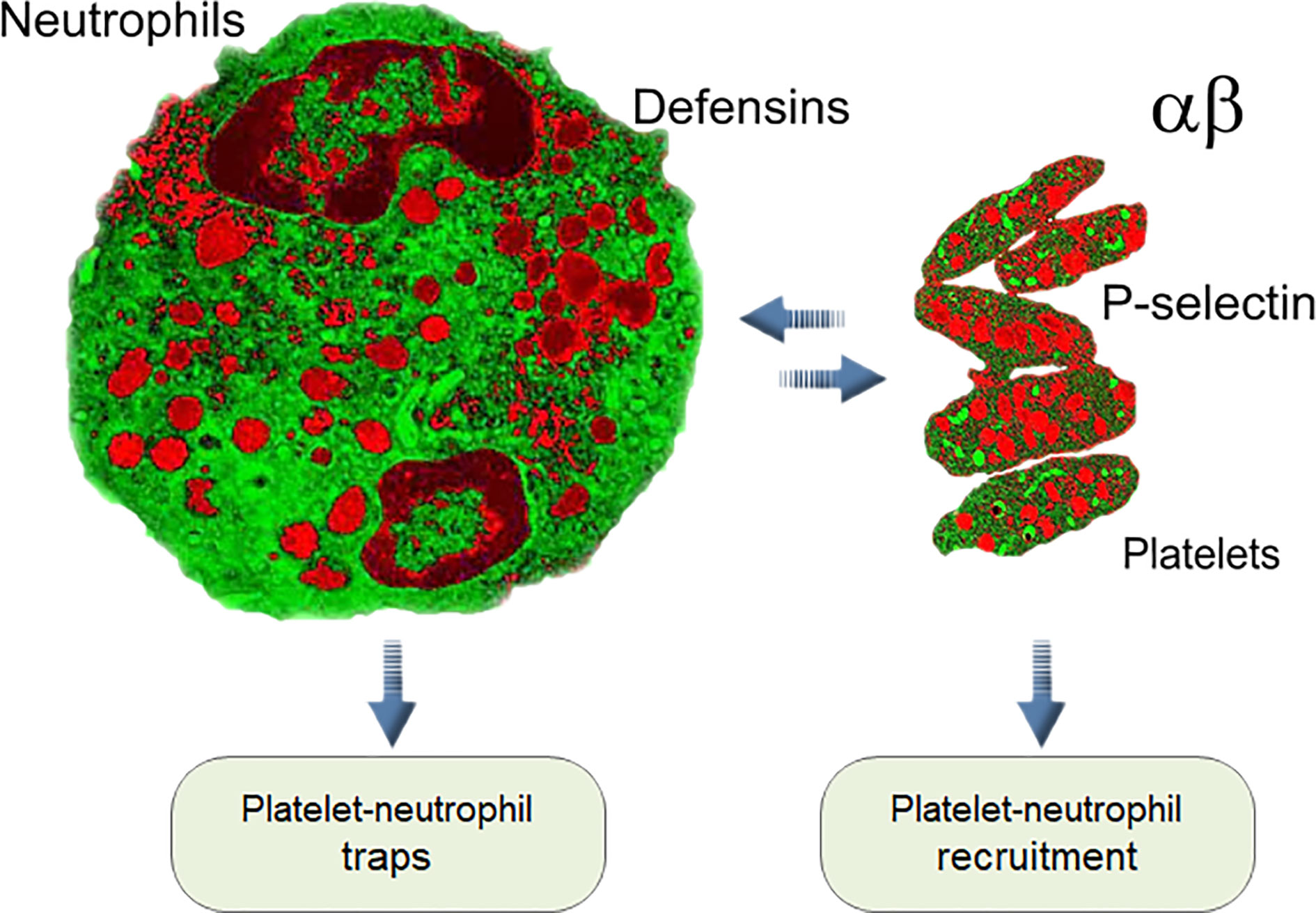
Frontiers On the Role of Platelet-Generated Amyloid Beta Peptides in Certain Amyloidosis Health Complications

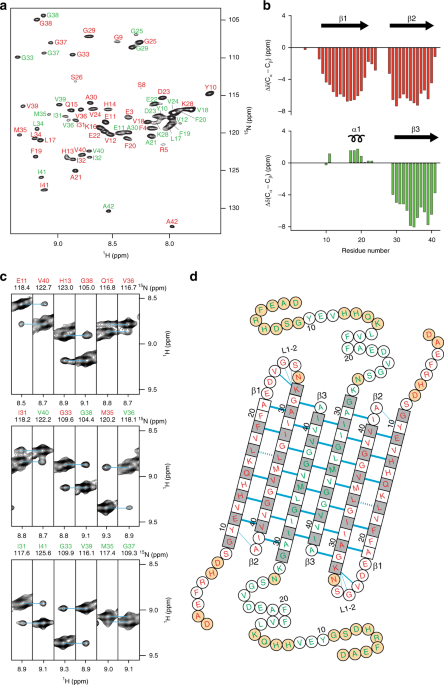
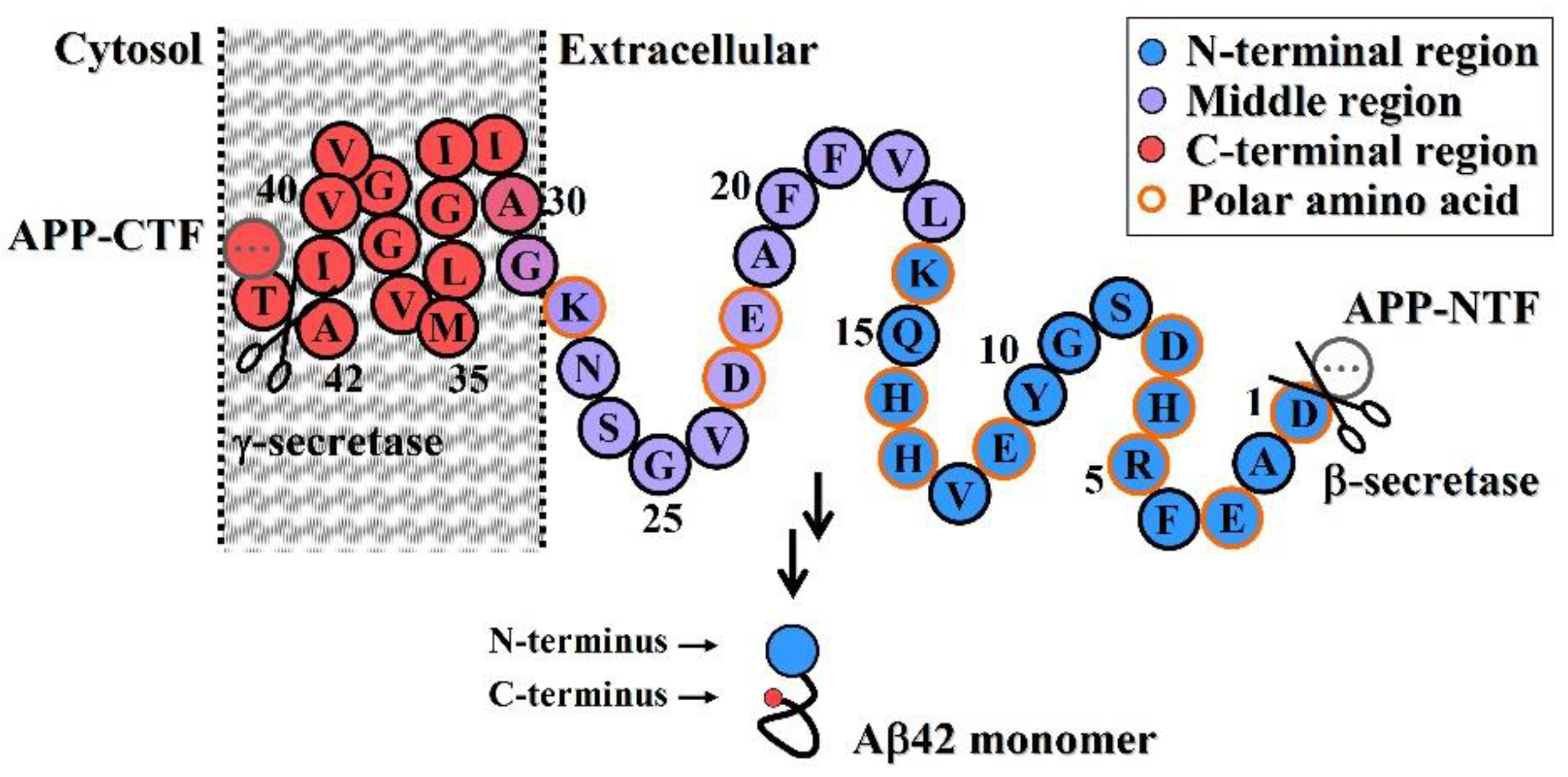
PDF] Alzheimer´s Disease-associated Aβ42 Peptide: Expression and Purification for NMR Structural Studies
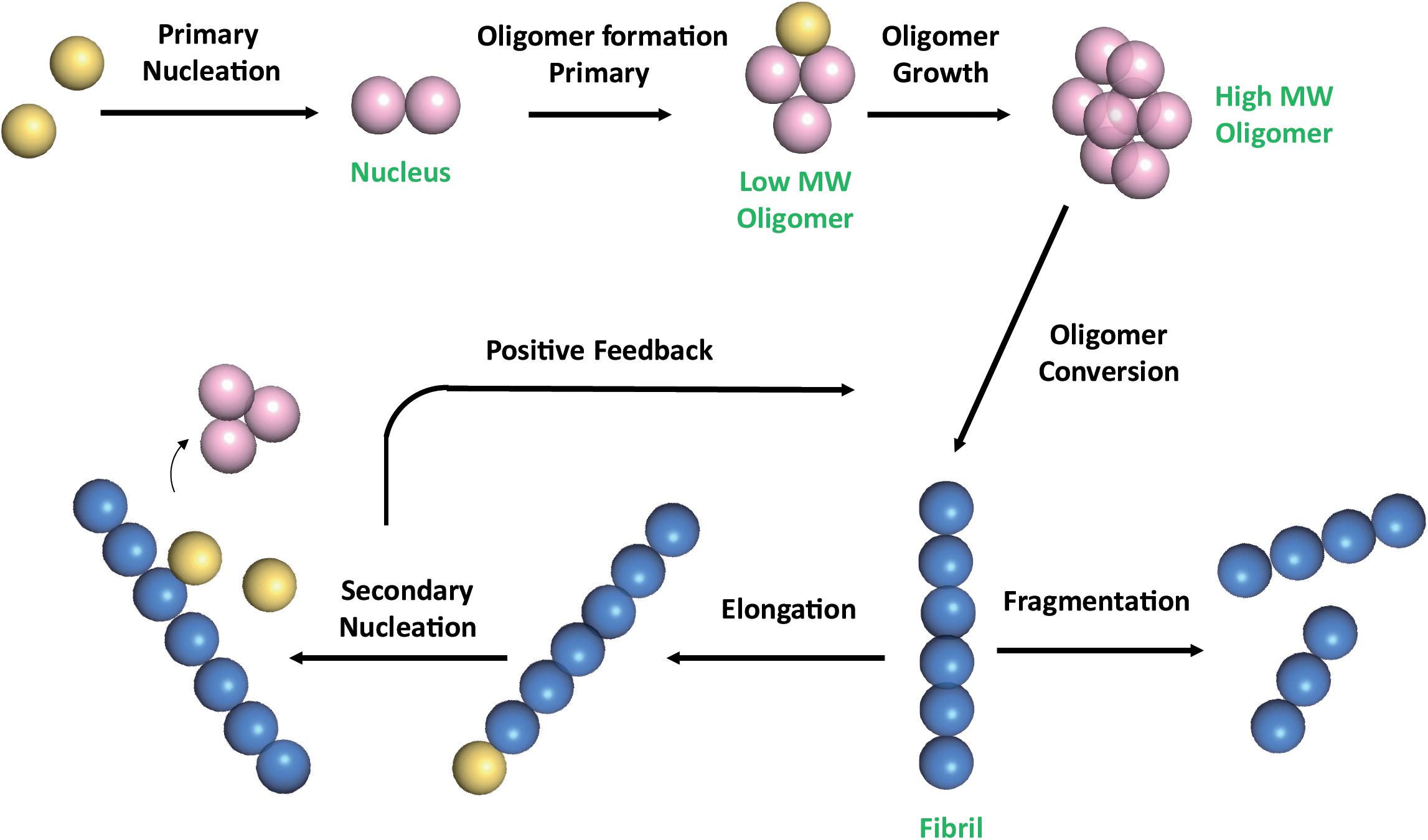
Frontiers Natural Compounds as Inhibitors of Aβ Peptide Aggregation: Chemical Requirements and Molecular Mechanisms

The structure of tyrosine-10 favors ionic conductance of

Exploring amyloid oligomers with peptide model systems - ScienceDirect

The amyloid-inhibiting NCAM-PrP peptide targets Aβ peptide aggregation in membrane-mimetic environments. - Abstract - Europe PMC
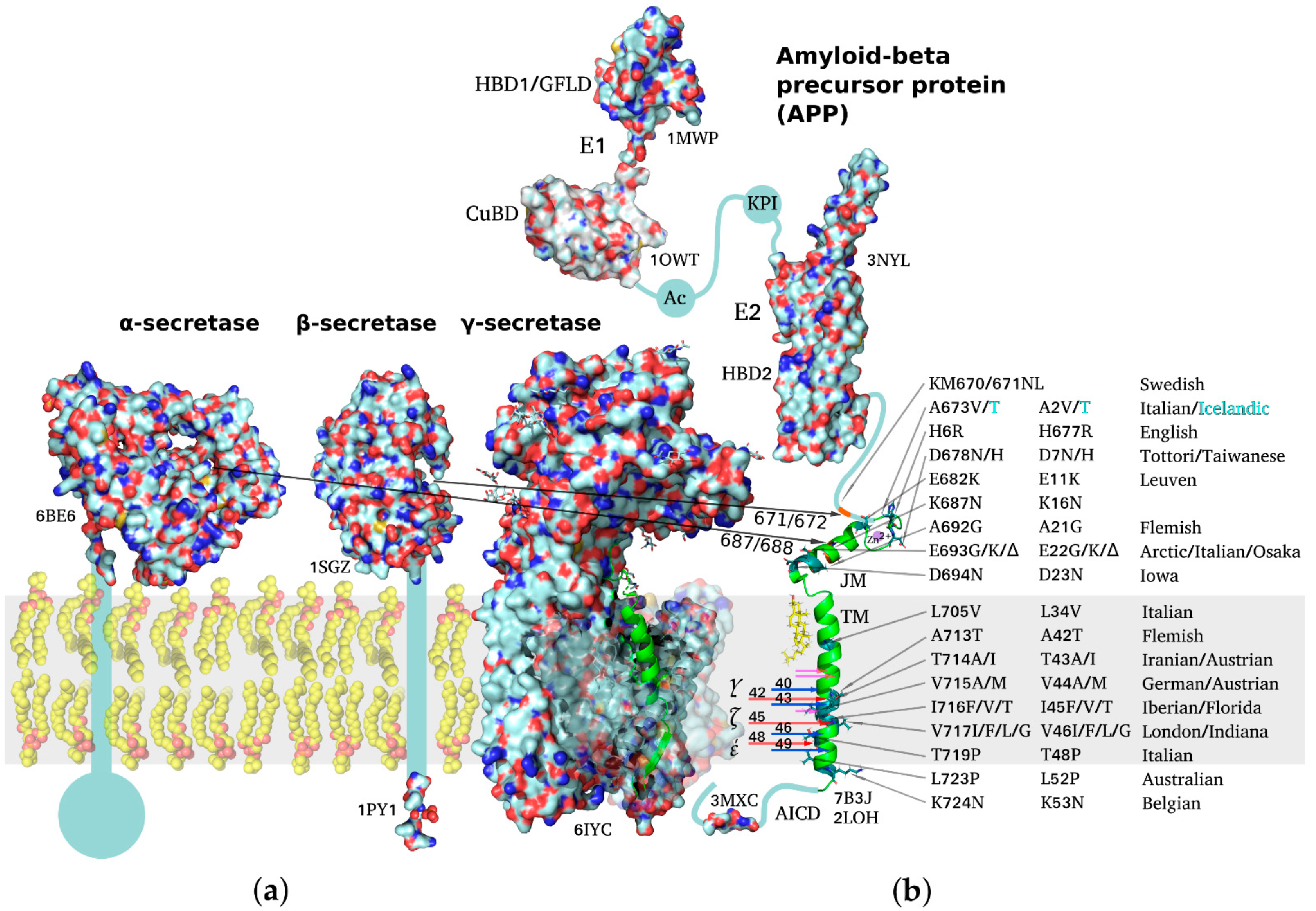
Molecules, Free Full-Text






